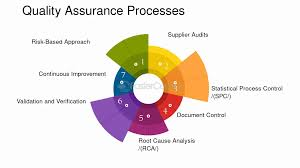
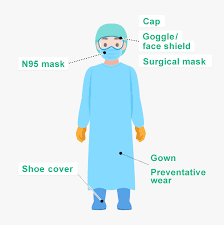
Medical supply companies are responsible for ensuring that the products they provide meet stringent quality and safety standards. This is crucial, as medical supplies are directly linked to patient care and health outcomes. Quality control begins with sourcing high-quality raw materials from trusted suppliers and manufacturers. Medical supply companies must comply with national and international regulations, such as the FDA’s guidelines, to ensure their products are safe for use in healthcare settings. Many companies undergo third-party testing to verify that their products meet the required standards for safety, durability, and efficacy.
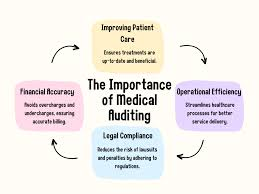
Once the products are manufactured, quality control measures are implemented at various stages of the production process. This includes inspecting individual items for defects, conducting rigorous testing on materials, and ensuring that packaging is intact to avoid contamination. Companies also perform regular audits of their suppliers and factories to ensure compliance with safety protocols. In addition, post-market surveillance is essential for identifying any potential issues with medical supplies once they are on the market. By maintaining a robust quality control system, medical supply companies can ensure that their products meet the highest safety standards, ultimately protecting both healthcare providers and patients.